Protected: PT-Studio: New Advanced Software for Tablet Hardness Testers
PT-Studio: New Advanced Software for Tablet Hardness Testers
Introducing WinDoc PT-Studio (or short PT-Studio), the new 21 CFR Part 11-compliant software for Pharma Test tablet hardness testing instruments. PT-Studio is an advanced, user-friendly, Windows-based platform designed to support precise pharmaceutical testing and robust data management. It is the successor of PTB32. Features that comply with 21 CFR Part 11 include an audit trail, electronic signatures, and user administration. Built to optimize workflow efficiency, ensure data integrity, and streamline validation processes, the software is an ideal solution for regulated environments where accuracy and compliance are paramount.
1. User interface
2. User, method and product administration
3. Audit trail and electronic signatures
4. Compatible models
5. Availability
1. User Interface
The software features an intuitive interface with streamlined navigation for user, method, and product administration. A central home screen enables test initiation, while the analysis window and tabs for reports and audit trail ensure efficient data handling and regulatory compliance.
2. User, Method and Product Administration
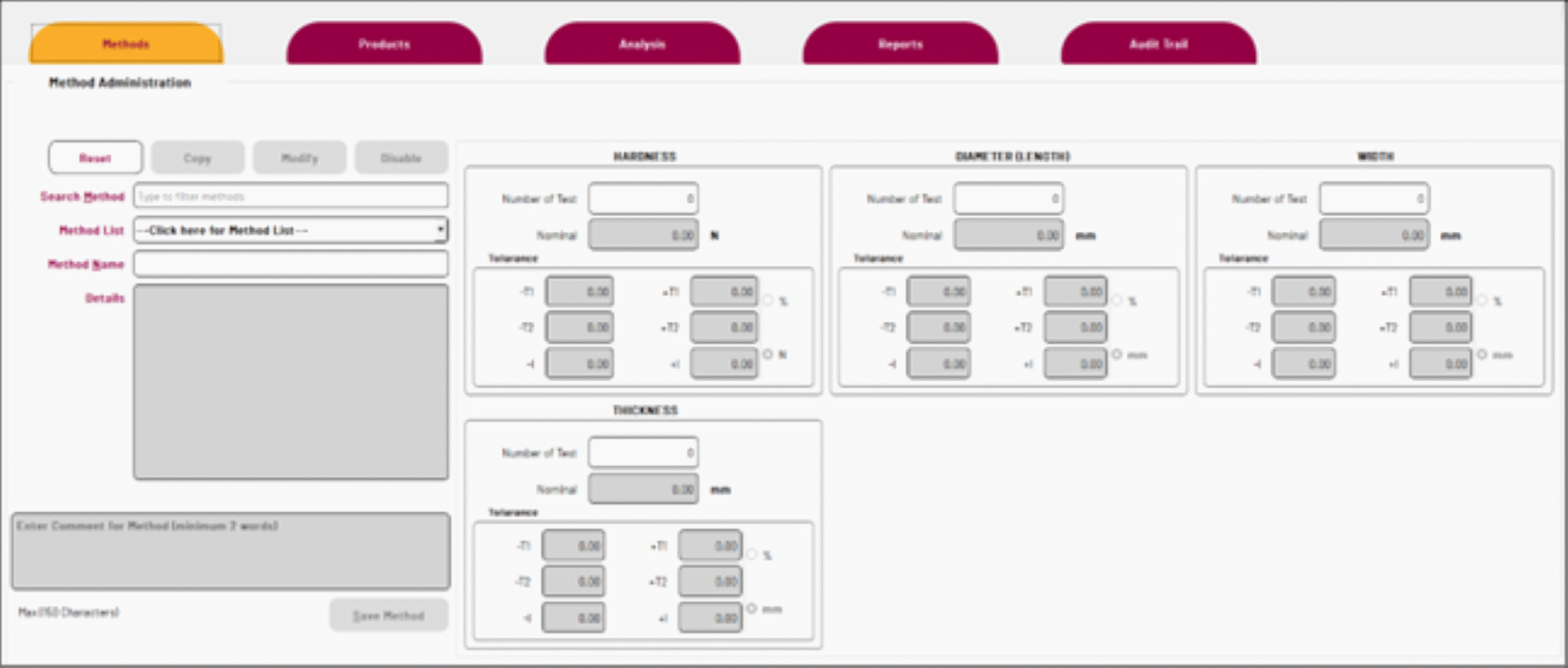
PT-Studio has a user administration module for managing user accounts, permissions, and security settings – ensuring data integrity. The method administration section efficient management of testing methods. Users can define method names, add comments, and configure parameters such as hardness, diameter, width, thickness and weight.
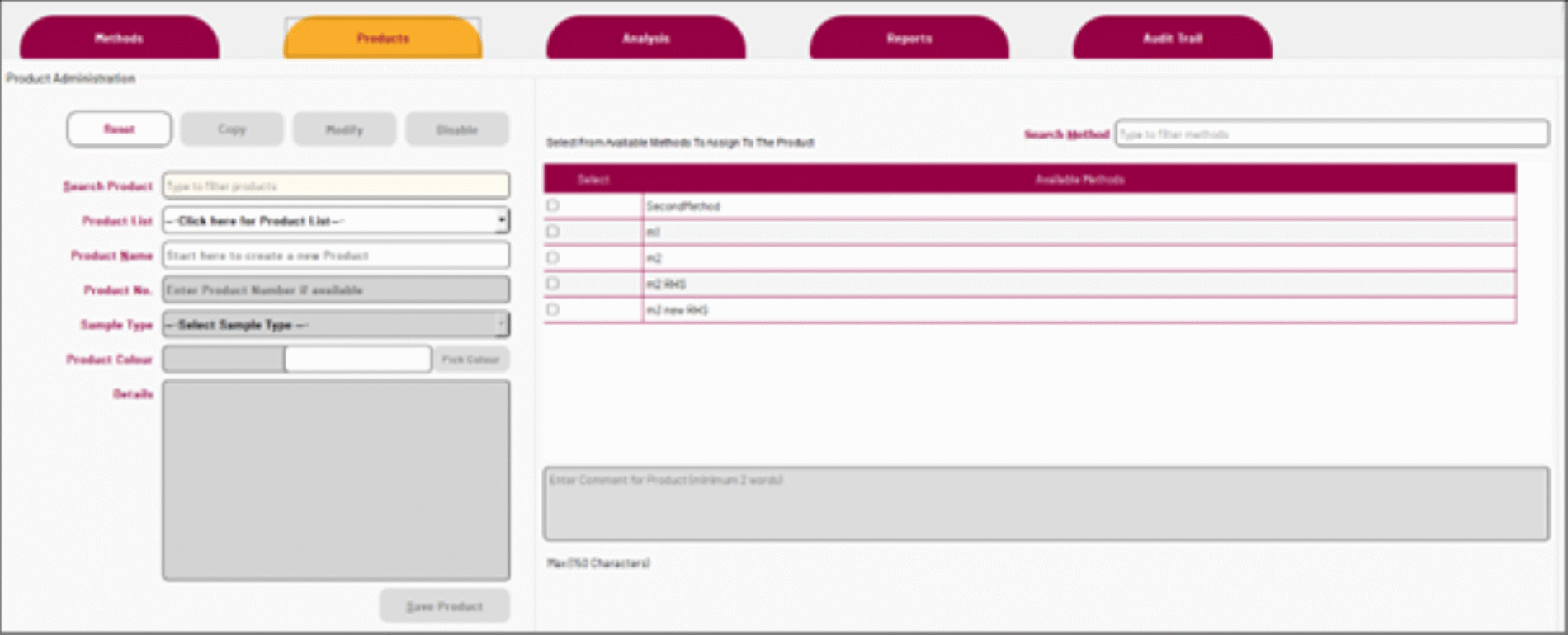
Product administration is a versatile tool that includes a search bar for filtering existing entries, dropdowns for selecting sample types, and input fields for product names, numbers, and details. Users can assign methods to products via a dedicated selection panel with search and table views for easy navigation.
3. Audit Trail and Electronic Signatures
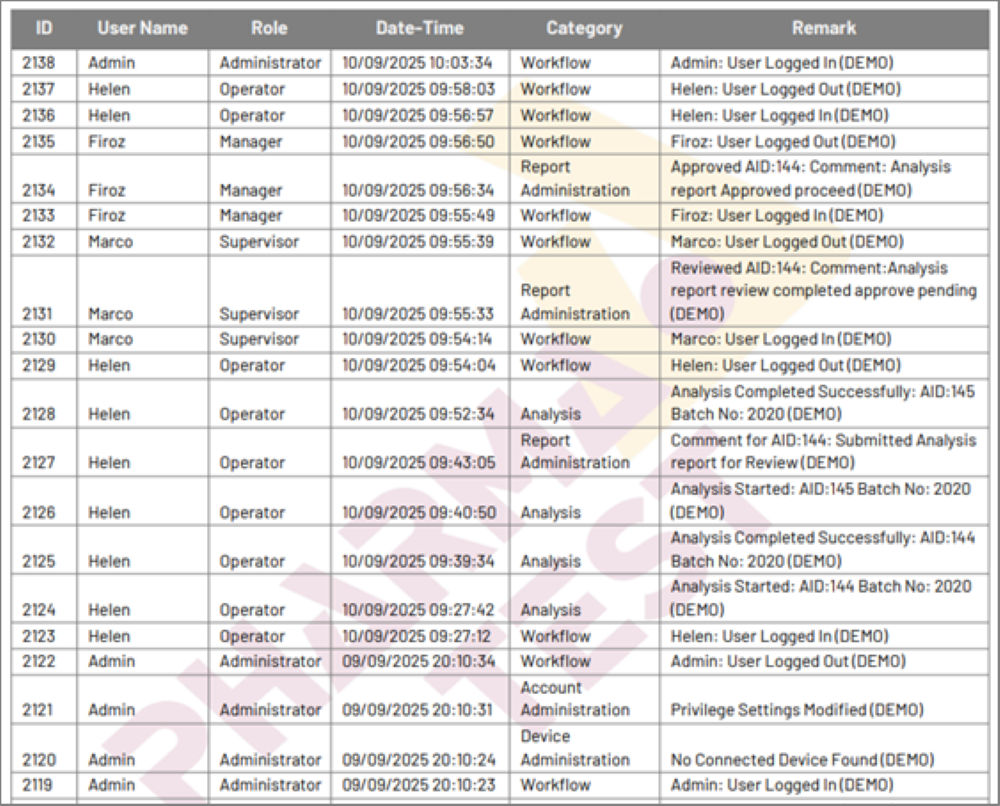
The audit trail feature in PT-Studio ensures 21 CFR Part 11 compliance and provides an efficient way to monitor and document user interactions within a system. It logs key activities such as logins, password changes, and logouts, along with timestamps, user roles, and event categories. It is an essential tool for maintaining transparency and accountability in digital environments. PT-Studio supports a three-level electronic signature system for all test reports, ensuring compliance with 21 CFR Part 11 and enabling traceability.
4. Compatible Models
The PT-Studio software is compatible with the following Pharma Test hardness tester models:
- PTB 330*
- PTB 430*
- PTB 500*
- PTB-M100
- PTB 111E/EP
- PTB 311E
- PTBA 211E
(* development is in progress)
5. Availability
WinDoc PT-Studio is available now! The PTB32 software will remain available.