Dissolution Tester
Tablet Dissolution Testing
A dissolution test is a means of identifying and proving the availability of active pharmaceutical ingredient (API) in their delivered form. A dissolution test reflects the availability of active substance and allows the prediction of the time for complete release of the material from the dosage form. This test plays an important role in product development, equivalence studies and for product compliance and release decisions. It is vital that the dissolution system provides accurate and reproducible results. All Pharma Test tablet dissolution testing instruments are fully USP and EP compliant. They use our MonoShaft™ tool system and include a full set of vessel and USP Apparatus 2 paddles. A full range of dissolution accessories is also available.

PT-Node
Network Adapter for Printing and Data Transfer
Details
PTWS 820-MA
8-Position USP/EP Tablet Dissolution Testing Instrument with Media Addition Station
Details
PT-DR
Dispersion Releaser – Release Apparatus for Nano- and Microformulations
Details
ADS-L 1420
Online automated dissolution testing system with 14+2 position bath.
Details
PTWS 1420
The new 14+2 station dissolution testing instrument – ideal for comparative studies such as biowaiver tests.
Details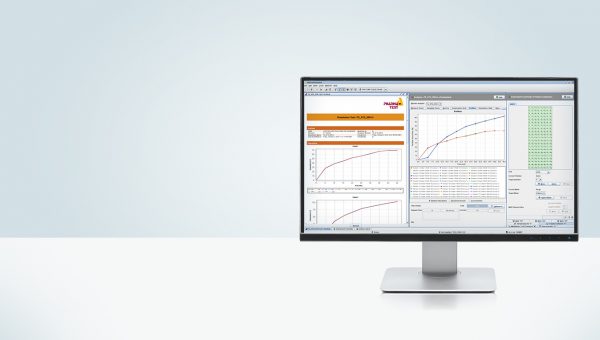
WinDiss ARGUS Dissolution Software
Details
PTWS 120D
The flexible dissolution bath for constrained bench space requirements.
Details
PTWS 120S
This 6 station tablet dissolution bath with individual stirring speed control is ideal for R&D purposes.
Details
PTWS 820D
The compact, 8-station tablet dissolution testing instrument.
Details
PTWS 620
The Magnificent Six. All Six Stations in One Line for Optimal Visibility.
Details
PTWS 1220
The ideal choice for Biowaiver studies and dissolution method development.
Details
PTWS D620
"Dual drive" tablet dissolution bath with independant stirring speed control for front and back row.
Details
